As Acidification Increases, Ocean Biodiversity May Decline

Contents
Dubbed “the evil twin of global warming,” ocean acidification is a growing crisis that poses a threat to both water-dwelling species and human communities that rely on the ocean for food and livelihood.
Since pre-industrial times, the ocean’s pH has dropped from 8.2 to 8.1—a change that may seem insignificant, but actually represents a 30 percent increase in acidity. As the threat continues to mount, the German research project BIOACID (Biological Impacts of Ocean Acidification) seeks to provide a better understanding of the phenomenon by studying its effects around the world.
BIOACID began in 2009, and since that time, over 250 German researchers have contributed more than 580 publications to the scientific discourse on the effects of acidification and how the oceans are changing.
The organization recently released a report that synthesizes their most notable findings for climate negotiators and decision makers. Their work explores “how different marine species respond to ocean acidification, how these reactions impact the food web as well as material cycles and energy turnover in the ocean, and what consequences these changes have for economy and society.”
Field research for the project has spanned multiple oceans, where key species and communities have been studied under natural conditions. In the laboratory, researchers have also been able to test for coming changes by exposing organisms to simulated future conditions.
Their results indicate that acidification is only one part of a larger problem. While organisms might be capable of adapting to the shift in pH, acidification is typically accompanied by other environmental stressors that make adaptation all the more difficult.
In some cases, marine life that had been able to withstand acidification by itself could not tolerate the additional stress of increased water temperatures, researchers found. Other factors like pollution and eutrophication—an excess of nutrients—compounded the harm.
Further, rising water temperatures are forcing many species to abandon part or all of their original habitats, wreaking additional havoc on ecosystems. And a 1.2 degree increase in global temperature—which is significantly under the 2 degree limit set in the Paris Climate Agreements—is expected to kill at least half of the world’s tropical coral reefs.
Acidification itself is a multipronged threat. When carbon dioxide is absorbed by the ocean, a series of chemical reactions take place. These reactions have two important outcomes: acid levels increase and the compound carbonate is transformed into bicarbonate. Both of these results have widespread effects on the organisms who make their homes in our oceans.
Increased acidity has a particularly harmful effect on organisms in their early life stages, such as fish larvae. This means, among other things, the depletion of fish stocks—a cornerstone of the economy as well as diet in many human communities. Researchers find that acidification and warming “work synergistically, especially on the most sensitive early life stages”—of fish, as well as embryo and larval survival.
Many species are harmed as well by the falling levels of carbonate, which is an essential building block for organisms like coral, mussels, and some plankton. Like all calcifying corals, the cold-water coral species Lophelia pertusa builds its skeleton from calcium carbonate. Some research suggests that acidification threatens both to slow its growth and to corrode the dead branches that are no longer protected by organic matter.
As a “reef engineer,” Lophelia is home to countless species; as it suffers, so will they. The BIOACID reports warns that to “preserve the magnificent oases of biodiversity founded by Lophelia pertusa, effects of climate change need to be minimised even now—while science continues to investigate this complex marine ecosystem.”
Even those organisms not directly affected by acidification may find themselves in trouble as their ecosystems are thrown out of balance. Small changes at the bottom of the food web, for example, may have big effects at higher trophic levels. In the Artic, Limacina helicina—a tiny swimming snail or “sea butterfly”—is a major source of food for many marine animals. The polar cod species Boreogadus saida, which feeds on Limacina, is a key food source for larger fish, birds, and mammals such as whales and seals.
As acidification increases, research suggests that Limacina’s nutrional value will decrease as its metabolism and shell growth are affected; its numbers, too, will likely drop. With the disappearance of this prey, the polar cod will likely suffer. Diminishing cod populations will in turn affect the many predators who feed on them.
Even where acidification stands to benefit a particular species, the overall impact on the ecosystem can be negative. In the Baltic Sea, BIOACID scientists have found that Nodularia spumigena, a species of cyanobacteria, “manages perfectly with water temperatures above 16 degrees Celsius and elevated carbon dioxide concentrations—whereas other organisms already reach their limits at less warming.”
Nodularia becomes more productive under acidified conditions, producing bacterial “blooms” that can extend upwards of 60,000 square kilometers in the Baltic Sea. These blooms block light from other organisms, and as dead bacteria degrade near the ocean floor they take up precious oxygen. The cells also release toxins that are harmful to marine animals and humans alike.
Ultimately biodiversity, “a basic requirement for ecosystem functioning and ultimately even human wellbeing,” will be lost. Damage to tropical coral reefs, which are home to one quarter of all marine species, could drastically reduce the ocean’s biodiversity. And as biodiversity decreases, an ecosystem becomes more fragile: ecological functions that were once performed by several different species become entirely dependent on only one.
And the diversity of marine ecosystems is not the only thing at stake. Currently, the ocean plays a major mitigating role in global warming, absorbing around 30 percent of the carbon dioxide emitted by humans. It also absorbs over 90 percent of the heat produced by the greenhouse effect. But as acidification continues, the ocean will take up less and less carbon dioxide—meaning we may see an increase in the rate of global warming.
The ocean controls carbon dioxide uptake in part through a biological mechanism known as the carbon pump. Normally, phytoplankton near the ocean’s surface take up carbon dioxide and then sink towards the ocean floor. This process lowers surface carbon dioxide concentrations, facilitating its uptake from the atmosphere.
But acidification weakens this biological carbon pump. Researchers have found that acidified conditions favor smaller types of phytoplankton, which sink more slowly. In addition, heavier calcifying plankton—which typically propel the pump by sinking more quickly—will have increasing difficulty forming their weighty calcium carbonate shells. As the pump’s efficiency decreases, so will the uptake of carbon dioxide from the air.
The BIOACID report stresses that the risks of acidification remain largely uncertain. However, despite—or perhaps because of— this, society must tread cautiously with care of the oceans. The report explains, “Following the precautionary principle is the best way to act when considering potential risks to the environment and humankind, including future generations.”
About the Future of Life Institute
The Future of Life Institute (FLI) is a global think tank with a team of 20+ full-time staff operating across the US and Europe. FLI has been working to steer the development of transformative technologies towards benefitting life and away from extreme large-scale risks since its founding in 2014. Find out more about our mission or explore our work.
Related content
Other posts about Climate & Environment, Recent News
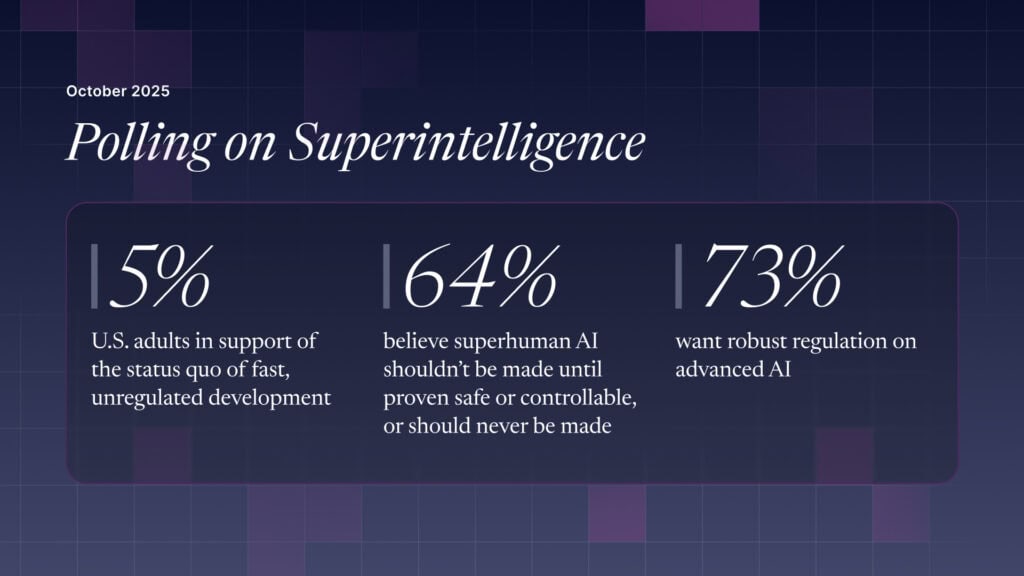
The U.S. Public Wants Regulation (or Prohibition) of Expert‑Level and Superhuman AI
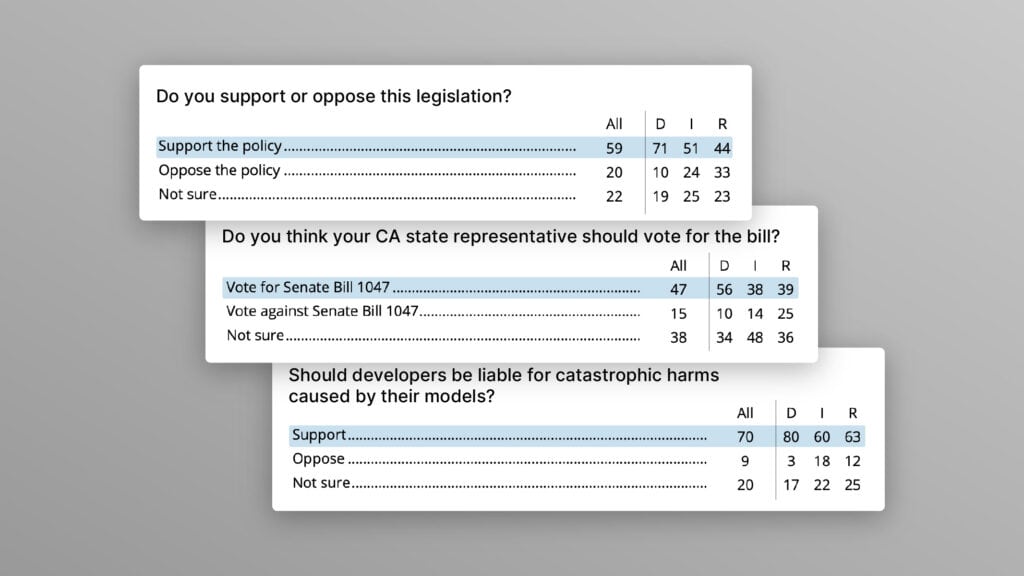
Poll Shows Broad Popularity of CA SB1047 to Regulate AI