The Federal Government Updates Biotech Regulations

Contents
Click here to see this page in other languages: Russian ![]()
By Wakanene Kamau
This summer’s GMO labeling bill and the rise of genetic engineering techniques to combat Zika — the virus linked to microcephaly and Guillain-Barre syndrome — have cast new light on how the government ensures public safety.
As researchers and companies scramble to apply the latest advances in synthetic biology, like the gene-editing technique CRISPR, the public has grown increasingly wary of embracing technology that they perceive as a threat to their health or the health of the environment. How, and to what degree, can the drive to develop and deploy new biotechnologies be reconciled with the need to keep the public safe and informed?
Last Friday, the federal government took a big step in framing the debate by releasing two documents that will modernize the 1986 Coordinated Framework for the Regulation of Biotechnology (Coordinated Framework). The Coordinated Framework is the outline for the network of regulatory policies that are used to ensure the safety of biotechnology products.
The Update to the Coordinated Framework, one of the documents released last week, is the first comprehensive review of how the federal government presently regulates biotechnology. It provides case-studies, graphics, and tables to clarify what tools the government uses to make decisions.
The National Strategy for Modernizing the Regulatory System for Biotechnology Products, the second recently released document, provides the long-term vision for how government agencies will handle emerging technologies. It includes oversight by the Food and Drug Administration (FDA), the U.S. Department of Agriculture (USDA), and the Environmental Protection Agency (EPA).
These documents are the result of work than began last summer when the Office of Science and Technology Policy (OSTP) announced a yearlong project to revise the way biotechnology innovations are regulated. The central document, The Coordinated Framework for the Regulation of Biotechnology, was last updated over 20 years ago.
The Coordinated Framework was first issued in 1986 as a response to a new gene-splicing technique that was leaving academic laboratories and entering the marketplace. Researchers had learned to take DNA from multiple sources and splice it together in a process called recombineering. This recombined DNA, known as rDNA, opened the floodgates for new uses that expanded beyond biomedicine and into industries like agriculture and cosmetics.
As researchers saw increasing applications for use in the environment, namely in genetically engineering animals and plants, concerns arose from a variety of stakeholders calling for attention from the federal government. Special interest groups were wary of the effect of commercial rDNA on public and environmental health; outside investors sought assurances that products would be able to legally enter the market; and fledgling biotech companies struggled to navigate regulatory networks.
This tension led the OSTP to develop an interagency effort to outline how to oversee the biotechnology industry. The culmination of this process created a policy framework for how existing legislation would be applied to various kinds of biotechnology. It coordinated across three responsible organizations: the Food and Drug Administration (FDA), the U.S. Department of Agriculture (USDA), and the Environmental Protection Agency (EPA).
Broadly, the FDA regulates genetically modified food and food additives, the USDA oversees genetically modified plants and animals, and the EPA tracks microbial pesticides and engineered algaes. By 1986, the first iteration of the Coordinated Framework was finalized and issued.
The Coordinated Framework was updated in 1992 to more clearly describe the scope of how federal agencies would exercise authority in cases where the established rule of law left room for interpretation. The central premise of the update was to look at the product itself and not the process by which it was made. The OSTP and federal government did not see new biotechnology methods as inherently risky but recognized that their applications could be.
However, since 1992, there have been a number of technologies that have raised new questions on the scope of agency authority. Among these are new methods for new applications, such as bioreactors for the biosynthesis of industrially important chemicals or CRISPR-Cas9 to develop gene drives to combat vector-borne disease. Researchers are also increasingly using new methods for old applications, such as zinc finger nucleases and transcription activator-like effector nucleases, in addition to CRISPR-Cas9, for genome editing to introduce beneficial traits in crops.
But what kind of risks do these innovations create and how could the Coordinated Framework be used to mitigate them?
In theory, the Coordinated Framework aligns a new innovation with the federal agency that has the most experience working in its respective field. In practice, however, making decisions between agencies with overlapping interests and experience has been difficult.
The recent debate over the review of a genetically modified mosquito developed by the UK-based start-up Oxitec to combat the Zika virus shows how controversial the subject can be. Oxitec’s genetically engineered a male Aedes aegypti mosquito (the host of Zika, along with dengue, yellow fever, and chikungunya viruses) with a gene lethal to offspring it has with wild female mosquitoes. The plan would be to release the genetically engineered male mosquitoes into the wild where they can mate with native female mosquitos and crash the local population.
Using older genetics techniques, this process would have needed approval from the USDA, which has extensive experience with insecticides. However, because the new method is akin to a “new animal drug,” its oversight fell to the FDA. And the FDA created an uproar when it approved field trials of the Oxitec technology in Florida this August.
Confusion and frustration over who is and who should be responsible in cases like this one have brought an end to the 20 year silence on the measure. In fact, the need to involve a greater amount of clarity, responsibility, and understanding in the regulatory approval process was reaffirmed last year. The OSTP sent a Memo last summer to the FDA, USDA and EPA announcing the scheduled update to the Coordinated Framework.
Since the Memo was released, the OSTP has organized a series of three “public engagement sessions” (notes available here, here and here) to explain how to the Coordinated Framework presently works, as well as to accept input from the public. The release of the Update to the Coordinated Framework and the National Strategy are two measures of accountability. The Administration will accept feedback on the measures for 40 days following a notice of request for public comment to be published by the Federal Register.
While scientific breakthroughs have the potential to spur wide-ranging innovations, it is important to ensure due respect is given to the potential dangers those innovations present.
You can sign up for updates from the White House on Bioregulation here.
Wakanene is a science writer based in Seattle, Wa. You can reach him on twitter @ws_kamau.
About the Future of Life Institute
The Future of Life Institute (FLI) is a global think tank with a team of 20+ full-time staff operating across the US and Europe. FLI has been working to steer the development of transformative technologies towards benefitting life and away from extreme large-scale risks since its founding in 2014. Find out more about our mission or explore our work.
Related content
Other posts about Biotech, Recent News
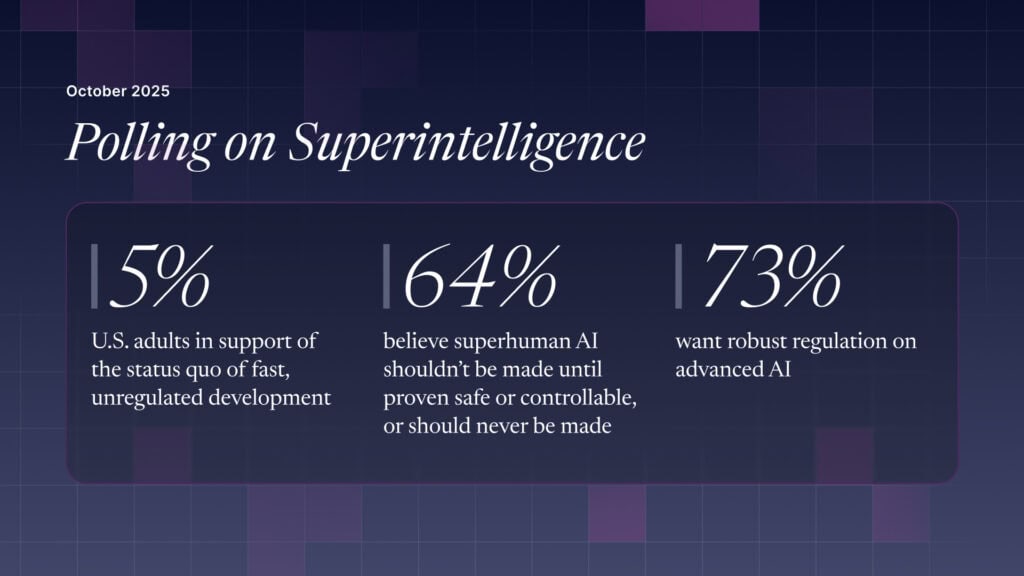
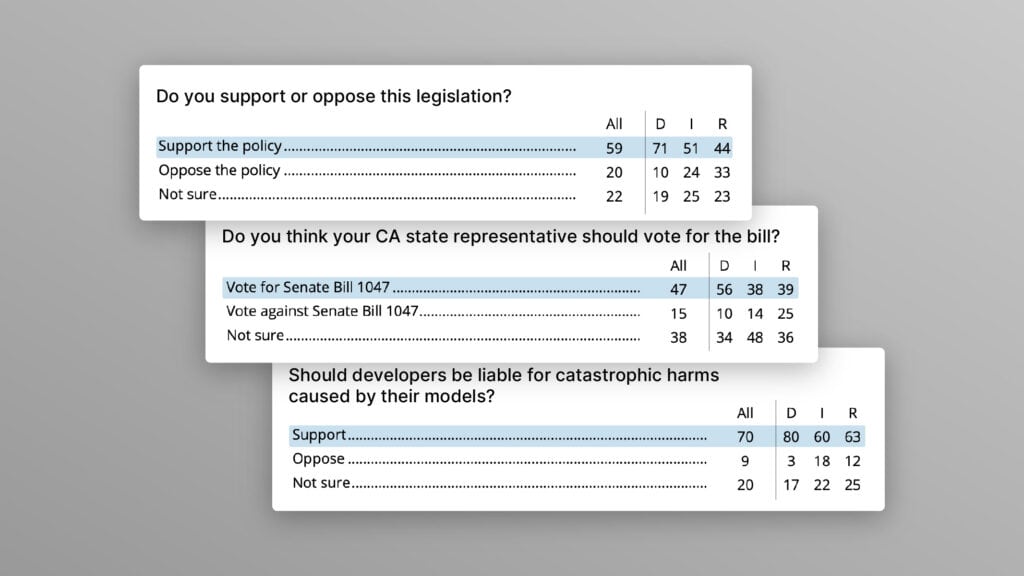
The U.S. Public Wants Regulation (or Prohibition) of Expert‑Level and Superhuman AI

AI Testing Mostly Uses English Right Now. That’s Risky